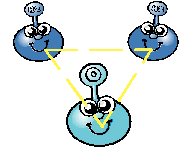
Hydrogen (H) is a very special type of atom. |
|
|
|
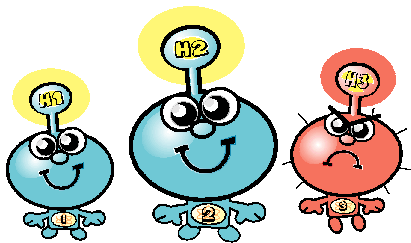
Hydrogen H3 has 1 proton an 2 neutrons in its core. This is the most aggressive member of the whole family, called Tritium. It can be very dangerous and most aggressive, because it is not stable - it is instable. The atoms of Tritium decay into other particles. By that Tritium sends rays into the atmosphere, which damage the health of everything living. The rays are so strong that they can even destroy our cells completely by intruding into each cell. |
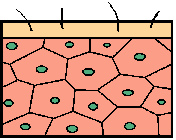
… our body, which is built by millions of cells, needs every cell to be healthy and strong. |
|
|